Digital health funding declines for the third year in a row
AI-enabled digital health startups raised $3.7B, 37% of total funding for the sector
Read more... Right now, there are three key problems facing the clinical trial space: making research more patient-centric; maximizing the impact of data on human lives; and better addressing the needs of underserved communities.
Right now, there are three key problems facing the clinical trial space: making research more patient-centric; maximizing the impact of data on human lives; and better addressing the needs of underserved communities.
Fixing those issues is the goal of Castor, a company that automates the research process.
"We saw that the life sciences industry was lacking a comprehensive and scalable solution for recruiting candidates for clinical trials, managing the research process, and effectively harnessing the vast amounts of data those clinical trials produce to drive medical breakthroughs," Derk Arts, MD, PhD, CEO and Founder of Castor, told me.
"We created Castor to address those pain points and streamline and optimize the clinical trial process."
On Wedneday, the company announced that it raised $12 million in funding. The round was led by Two Sigma Ventures with participation from Hambrecht Ducera Growth Ventures and existing investor INKEF Capital. This round brings its total funding to $18.25 million, after a $6.25 seed round in 2018, led by INKEF Capital.
The typical customer for Castor are medical device, biotech, and pharmaceutical companies and contract research organizations, of which is now has 192 total customer. The company also more than 50,000 customers across academia and commercial research, powering more than 4,000 studies with more than 2,000,000 enrolled patients across 90 countries in areas such as diabetes, cardiovascular disease, rare diseases, infectious diseases, and oncology.
Through its technology, the company is able to reduce trial throughput time by up to 45 percent by allowing its customers to deploy and configure their own projects through its platform, recruit patients through online channels, rather than traditional face to face site visits, and reuse existing data, which also reduces the need for slow, manual source data verification.
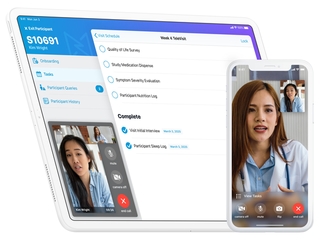
"Castor’s easy-to-use cloud-based platform simplifies the clinical trial process, from recruitment to analysis. Researchers leverage the platform to run remote clinical trials and capture data from patients, physicians, EHR systems and wearables. The platform transforms this data into a machine readable format that facilitates the use of AI and makes large scale sharing and reuse of clinical trial data possible for the first time," explained Arts.
Castor's technology and platform can also be used to help combat COVID-19; more than 200 COVID-19 projects across 33 countries are currently running on the platform, including the World Health Organization's global Solidarity trial.
Through the Castor platform, more than 10,000,000 COVID-19 data points have already been captured, and 50 COVID-19 projects have committed to making their data reusable and accessible to others.
"We’re at the forefront of fighting COVID-19. As one of the only providers that can enable large-scale decentralized trials to accelerate the work of researchers who are trying to combat the disease, we made our platform freely available for all non-profit COVID-19 research starting in February," Arts said.
Castor says it will use this new funding to strengthen its patient-facing technology, which includes video-enabled consent to remotely enroll patients in clinical trials. The company, which is based in the United States and The Netherlands, will also continue its U.S. expansion, which includes building outs it team, while also using the new money to "build up our commercial customer base among biopharma and medical device companies."
"Our mission is to bring medical research into 21st century by helping researchers efficiently run clinical trials, capture and reuse data, and make data reusable to enable more medical discovery," said Arts.
(Image source: castoredc.com)
AI-enabled digital health startups raised $3.7B, 37% of total funding for the sector
Read more...OXcan combines proteomics and artificial intelligence for early detection
Read more...Nearly $265B in claims are denied every year because of the way they're coded
Read more...