 AVS (Amplitude Vascular Systems) is a company creating a new treatment option for pulsatile intravascular lithotripsy (PIVL), an emerging, minimally invasive treatment for vascular calcification, a common condition that has been shown to increase the risk of major cardiovascular events, such as heart attacks, strokes, or blood clots, and can lead to limb amputations. While the presence of coronary artery calcification is age and gender-dependent, it has been found to be present in 90% of men and 67% of women older than the age of 70.
AVS (Amplitude Vascular Systems) is a company creating a new treatment option for pulsatile intravascular lithotripsy (PIVL), an emerging, minimally invasive treatment for vascular calcification, a common condition that has been shown to increase the risk of major cardiovascular events, such as heart attacks, strokes, or blood clots, and can lead to limb amputations. While the presence of coronary artery calcification is age and gender-dependent, it has been found to be present in 90% of men and 67% of women older than the age of 70.
Now the company has some fresh funding to help get that system into the hands of the people who need it the most.
On Tuesday, AVS announced that it raised an additional $8.8 million in Series B funding, adding onto the $20 million round it raised in January. The new funding came from BioStar Capital, who also led the previous tranche, along with CUE Growth Partners.
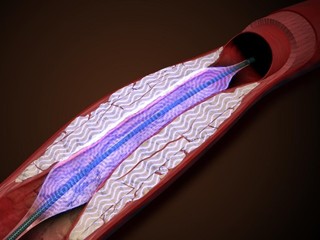
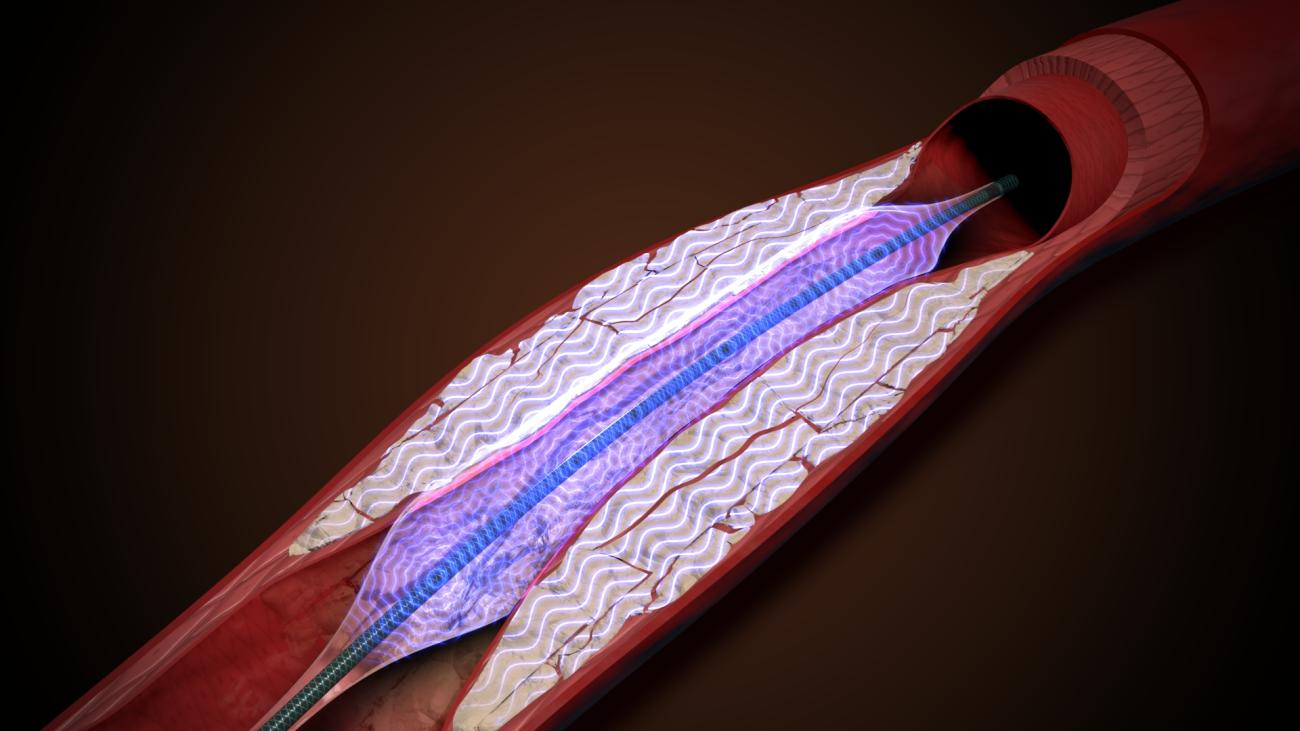
While the typical treatment for plaque-clogged blood vessels is either medication or lifestyle changes, such as eating healthier and exercising, it may also require vascular surgery, which comes with its own set of risks for the patient. With PIVL, however, multiple emitters mounted on a traditional catheter platform deliver localized pulsatile sonic pressure waves that are meant to break up vascular calcium.
AVS’s device is a balloon-based platform, called the PULSE IVL System, which is able to shatter calcium with pressure waves in frequent bursts while expanding calcified arteries. It utilizes low intensity, high frequency pressure waves to generate fractures in the plaque and restore blood flow.
The Pulse IVL System is still in development, and has not yet been cleared for commercial distribution, but the company has been showing its effectiveness through clinical trials; in September 2022, AVS announced enrollment, successful treatment, and positive 30-day follow-up data of the first patients in its POWER PAD I clinical trial. Earlier this year, AVS completed a 30-day follow-up with those clinical trial participants, who reported no major medical events.
The new funding will be used to continue to support the company’s clinical trials, while also advancing preclinical work for an expanded indication in coronary cases.
“We are aiming to build clinical evidence for an indication to treat coronary arterial calcification after gaining regulatory clearance for peripheral arterial calcification. We want to provide a new treatment option for coronary cases because it has a significant patient population and obvious need for new therapies,” Mark Toland, Executive Chairman and CEO of AVS, told VatorNews.
“It is estimated that 90 percent of men and 67 percent of women over age 70 have coronary artery calcification and heart disease remains the most common cause of death in the U.S. Our early trial results showed tremendous promise in successfully treating peripheral artery cases and we believe we can achieve similar results in coronary cases.”
The company’s roadmap includes completing its peripheral clinical trials and obtaining approval from the FDA. In addition, it will initiate early clinical trials for its coronary product.
“Intravascular lithotripsy is an emerging treatment paradigm for calcified arterial disease. Our PULSE IVL System uses a novel method to shatter calcium with pressure waves in frequent bursts and expands calcified arteries, all with a single device. We believe our platform can improve access to high-level care for patients in this disease state,” Toland said.
(Updated with comment from Mark Toland, Executive Chairman and CEO of AVS)
(Image source: avspulse.com)