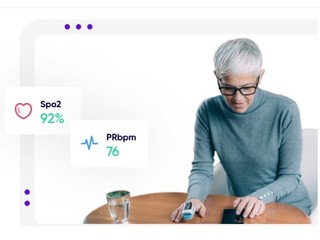
Now the company is using its technology and platform to help power clinical trials for Every Cure, a non-profit that looks to identify new uses for existing drugs and conduct clinical trials of the most promising treatments.
The two companies announced a new partnership on Wednesday to help speed up cures for the roughly 9,000 diseases that don’t have an FDA-approved treatment. Through this partnership, Medable will provide Every Cure with specialized software and services to conduct remote clinical trials for drug repurposing candidates, with the ultimate goal of getting FDA-approval for an existing drug with a new indication.
Every Cure, with officially launched in September in partnership with the Clinton Global Initiative, was co-founded by Dr. David Fajgenbaum, who was diagnosed with Castleman Disease in 2010 while he was in medical school.
Castleman disease is a condition in which benign growths form in lymph node tissue, and it had no known cure and no drugs in development at the time. However, Dr. Fajgenbaum was able to identify an existing drug, sirolimus, that could be repurposed to treat Castleman Disease; his team has since repeated this approach of identifying new uses for approved treatments 10 more times for Castleman disease, cancer, and COVID-19.
Every Cure is able to do this through a tool it developed called the MATRIX, which uses machine learning and AI to identify and rank promising treatments. A pilot study conducted by Every Cure uncovered 106 promising drug repurposing opportunities in the first 147 diseases.
Founded in 2015, Medable’s platform offers its clients, which include clinical trial sponsors, such as pharma and biotech companies, as well as clinical research organizations who manage trials for those companies, features such as patient identification and site selection through AI and machine learning; digitized patient enrollment; and the ability to do trials in any country and in any language. The company also also provides them with remote patient monitoring, and the ability to see streaming data and patient data in real-time.
The company also provides them with remote patient monitoring, and the ability to see streaming data and patient data in real-time.
“David Fajgenbaum’s story is an inspiration, and it reinforces the critical need for innovation in clinical research,” Dr. Michelle Longmire, CEO and co-founder of Medable, said in a statement.
“We live in an era of exponential improvements in medicine, but traditional clinical trials are a bottleneck in the system – and only yield 50 new FDA-approved treatments per year. With new technologies and new research techniques, we believe it’s possible to drastically increase the number of therapies in development – and Every Cure will be an important leader in driving that change.”