Walgreens partners with the government on decentralized clinical trials
The company will help BARDA validate, pilot and implement new products, technologies and approaches
 Decentralized clinical trials (DCTs) became the standard during COVID: as the country was in lockdown and having people come to participate in person was an unnecessary risk; it was safer and easier to conduct trials remotely. As the pandemic receded, and people could go in person again, DCTs were no longer the default, but that doesn't mean things have gone back to the way they were: they were projected to increase by 17% by the end of 2023, following a 28% increase in 2022 from 2021, and a 93% increase from 2020.
Decentralized clinical trials (DCTs) became the standard during COVID: as the country was in lockdown and having people come to participate in person was an unnecessary risk; it was safer and easier to conduct trials remotely. As the pandemic receded, and people could go in person again, DCTs were no longer the default, but that doesn't mean things have gone back to the way they were: they were projected to increase by 17% by the end of 2023, following a 28% increase in 2022 from 2021, and a 93% increase from 2020.
The government has also made them a priority, as the Biomedical Advanced Research and Development Authority (BARDA) launched the Decentralized Clinical Operations for Healthcare and Research (D-COHRe) program last year. And now BARDA has recruited Walgreens as a new partner to strengthen its model and reporting.
The partnership will use Walgreens’s clinical trial ecosystem, which has has reached more than five million patients to potentially recruit into clinical trials since its launch in 2022, to enhance decentralized clinical research in order to validate, pilot and implement new products, technologies and approaches for decentralized trials during a public health emergency. That includes immunizations, diagnostics, and treatments.
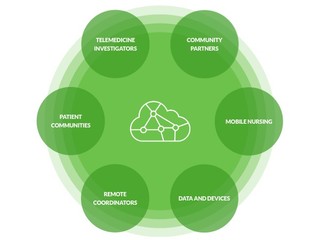
The D-COHRe program leverages public-private partnerships to enhance decentralized clinical capabilities and help bolster national preparedness for future health security threats.
On its website, the program says it's seeking multiple partnerships with different types of organizations who are interested in building or enhancing their clinical study capabilities, such as retail clinics, pharmacy clinics, mobile clinics, urgent care clinics, standalone ERs, telehealth providers and platforms, virtual health service providers, and CROs focused on decentralized clinical trials.
"The COVID-19 pandemic catalyzed decentralized care through increased use of telemedicine, retail/pharmacy clinic services, and other remote clinical care services. However, it also highlighted challenges in conducting clinical trials for medical countermeasure. Developers had difficulty working with sites to recruit, enroll, and retain patients especially in settings outside the hospital," D-COHRe wrote.
"D-COHRe aims to leverage the shift in decentralized care and enhance clinical study capabilities by partnering with organizations focused on providing decentralized clinical care, directly to where the patients seek it."
Walgreens and D-COHRe will also work to make decentralized clinical trials more accessible and representative of the U.S. population; currently, nearly 80% of trials fail to meet their enrollment goals, and only 5% of the U.S. population participates in clinical trials.
The two organizations has previously partnered: in July, Walgreens received a project award, valued up to $25 million, through the Rapid Response Partnership Vehicle to conduct a Phase IV observational clinical study focused on assessing Correlates of Protection, or responses to a vaccine that predict how well a vaccinated person will be protected from future infections, using COVID-19 vaccine data.
“It is a privilege to continue our partnership with BARDA to strengthen clinical research in the U.S. through a decentralized model in a community pharmacy setting like Walgreens,” Ramita Tandon, chief clinical trials officer at Walgreens, said in a statement.
“Our network of community pharmacies and our compliant and secure clinical trial platform enables us to pioneer a comprehensive solution to make clinical research an integral part of a patient’s healthcare journey, especially when it is most critical for the well-being of our country, during a public health emergency.”
(Image source: drive.hhs.gov)
Related News


Walgreens and RxSense partner on a tool for lower drug prices

ObvioHealth CEO Ivan Jarry on VatorNews podcast