Medable partners with Masimo to use its pulse oximeter in clinical trials
The MightySat Rx has already been used in eight trials for both lung and breast cancer
 Wearable devices have become essential to clinical research, as they enable research studies to collect health-related data on patients around the clock. These devices, which includes commercial fitness trackers that collect mobility and vital sign data, are especially important for decentralized trials, meaning ones where some, or sometimes all, activities occur at locations outside of a traditional clinical trial site, such as the ones being conducted by digital clinical trial platform Medable.
Wearable devices have become essential to clinical research, as they enable research studies to collect health-related data on patients around the clock. These devices, which includes commercial fitness trackers that collect mobility and vital sign data, are especially important for decentralized trials, meaning ones where some, or sometimes all, activities occur at locations outside of a traditional clinical trial site, such as the ones being conducted by digital clinical trial platform Medable.
Now the company will be incorporating a new set of wearables, having announced a new partnership with medical technology and automation solutions provider Masimo on Wednesday.
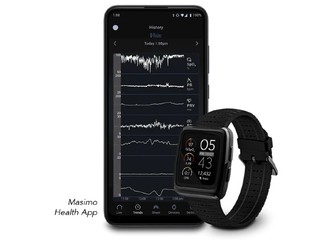
Through this collaboration, Medable has already begun to integrate Masimo’s MightySat Rx pulse oximeter into its platform for eight big-pharma-sponsored clinical trials spanning 25 countries, including over 3,000 patients, across two oncology indications: lung and breast cancer.
Medable offers its clients, which include clinical trial sponsors, such as pharma and biotech companies, as well as clinical research organizations who manage trials for those companies, features that include patient identification and site selection through AI and machine learning; digitized patient enrollment; and the ability to do trials in any country and in any language. It also provides them with remote patient monitoring, and the ability to see streaming data and patient data in real-time.
The company has deployed its software-as-a-service platform in more than 300 decentralized and hybrid clinical trials in 60 countries, serving more than one million patients and research participants globally. Its customers have seen 200% faster enrollment and 50% cost reductions.
Medable’s platform has been deployed in more than 300 trials in 60 countries and 120 languages. Currently, 20% of trials leveraging Medable’s evidence generation platform include a wearable device, and 35% of those are in oncology.
By incorporating Masimo's wearables, Medable will be enable both subjective data capture, via humans through its eCOA+ solution, and objective data capture via Masimo’s connected sensors.
“Medable has always been a leader in digital and decentralized clinical trials, so we are excited to work with a forward-thinking organization with the ambition and ability to transform research,” Dr. Daniel Cantillon, Chief Medical Officer at Masimo, said in a statement.
“Clinical trials require top-in-class accuracy and data quality both on-site and off-site from clinical offices. Medable is solving this problem through its advocacy and by empowering more patients to participate in research. And, Medable’s flexible platform enables scalability and data aggregation for a holistic approach.”
Founded in 2015, Medable raised four funding rounds in 18 months, including $304 million in October 2021, which valued the company at over $2 billion.
(Image source: masimo.com)
Related News