 Trump touts plasma treatment for COVID, FDA apologizes
Trump touts plasma treatment for COVID, FDA apologizes
President Donald Trump announced emergency authorization to treat COVID-19 patients with convalescent plasma taken from patients who have recovered from the coronavirus,, calling it “a breakthrough.”
However, so far, the evidence as to whether or not it works has not been conclusive, including when to administer it and what dose is needed.
“COVID-19 convalescent plasma should not be considered a new standard of care for the treatment of patients with COVID-19. Additional data will be forthcoming from other analyses and ongoing, well-controlled clinical trials in the coming months,” Denise Hinton, the chief scientist for the FDA, wrote in a letter describing the emergency authorization.
The announcement, which came on the eve of the Republican National Convention, was quickly accused of being politically motivated, and received pushback from medical experts.
FDA Commissioner Stephen Hahn also apologized for overstating the potential benefits of the treatment after saying that 35 more people out of 100 would survive the coronavirus if they were treated with the plasma, which is not what the initial results had shown.
“I have been criticized for remarks I made Sunday night about the benefits of convalescent plasma. The criticism is entirely justified. What I should have said better is that the data show a relative risk reduction not an absolute risk reduction,” Hahn tweeted.
 CDC changes testing guideline, but states push back
CDC changes testing guideline, but states push back
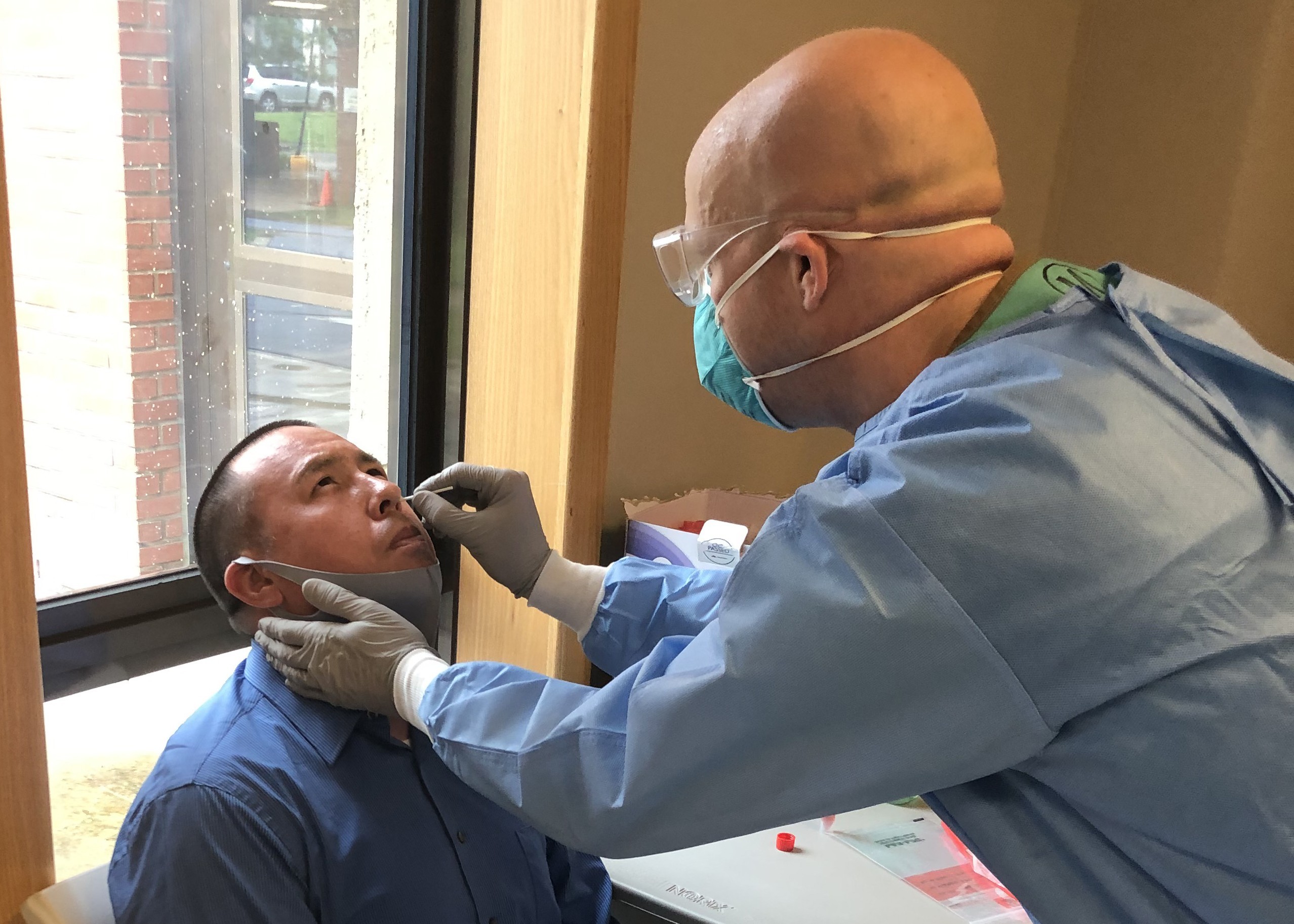
The Centers for Disease Control and Prevention changed its guidance on COVID-19 testing, stating that healthy people who have been exposed to COVID-19 "do not necessarily need a test" if they don't have any symptoms of the virus. Vulnerable individuals, including as older people and those with chronic medical conditions, are still recommended to get tested.
The CDC had previously recommended that anyone in close contact with someone who is infected get tested. An exposure is considered spending more than 15 minutes with an infected person at a range closer than 6 feet.
The change was met with criticism from infectious disease experts.
 Healthcare has been hot button a political issue for decades, with fights over Medicare going back to the mid-60s. The issue has been especially fractious over the last decade with the implementation of the Affordable Care Act, which passed without a single Republican voting for it.
Healthcare has been hot button a political issue for decades, with fights over Medicare going back to the mid-60s. The issue has been especially fractious over the last decade with the implementation of the Affordable Care Act, which passed without a single Republican voting for it.

