When a patient is put on a mechanical ventilator, their respiratory muscles start to atrophy, or waste away, within hours of being intubated, which advances rapidly, particularly during the first week of ventilation. All of this ultimately prolongs the duration of ventilation.
When a patient is put on a mechanical ventilator, their respiratory muscles start to atrophy, or waste away, within hours of being intubated, which advances rapidly, particularly during the first week of ventilation. All of this ultimately prolongs the duration of ventilation.
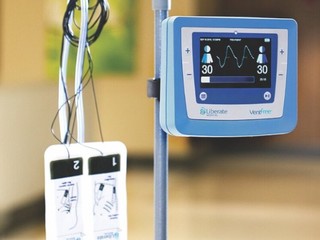
Liberate Medical is looking to change that by developing a non-invasive neurostimulation device, called VentFree, which is focused on transforming respiratory care, so that patients spend less time on a ventilator. Its solution is meant to prevent muscle weakness that didn’t rely on patient active participation.
“In the case of the expiratory muscles, the muscles Liberate’s VentFree devices targets, prior research has shown that weakness is associated with a 5x increase in the likelihood of failed extubation, or removal, from the ventilator,” Angus McLachlan, CEO of Liberate Medical, explained to VatorNews.
“More traditional methods of breathing muscle training, for example blowing against a resistance, and breathing exercises, all require the patient’s active participation, which is not possible in the ICU where patients are commonly sedated or delirious.”
On Wednesday, the company announced a $6.2 million Series B financing round co-led by a large strategic investor and Scientific Health Development, who will both join the board at Liberate Medical. With this round the company has raised $11.2 million in total in private financing, with $2.7 million in addition from grants.
The VentFree stimulator, applies non-invasive neuromuscular electrical stimulation in synchrony with a patient’s exhalation to cause the abdominal muscles, which are the major expiratory muscles, to contract without require active patient participation.
“This is like exercising the muscles while the patient is ventilated so that they don’t waste away,” said McLachlan.
“The main differentiating factors of our solution versus others in development is that ours is the only one targeting the expiratory muscles, as opposed to the inspiratory muscles, and that ours is non-invasive.”
The new funding will largely go toward supporting the clinical trial of VentFree, known as the PREVENT trial. The company is looking to prove that VentFree reduces the duration of invasive mechanical ventilation in patients that are at risk of being difficult to wean. This trial follows two successful pilot randomized controlled trials that were completed in Europe and Australia, though the company hasn’t shared details of the trial publicly yet.
So far, VentFree has FDA Breakthrough Device Designation, FDA Emergency Use Authorization and CE marking in the European Union. The PREVENT Trial will be the next step in Liberate Medical receiving FDA regulatory clearance so it can fully launch VentFree commercially.
“Our goal is to majorly improve the standard of care for patients with respiratory indications with innovative first of a kind solutions. Success for us means developing solutions clinically proven to improve patient outcomes and widespread adoption of our products. From this foundation, we believe financial success will follow,” said McLachlan.
(Image source: liberatemedical.com)