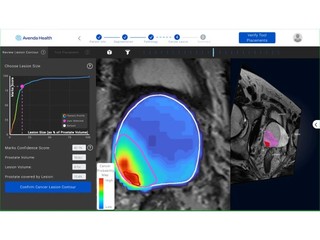
Avenda Health is a company that creates personalized prostate cancer treatment. Its cloud-based platform, iQuest, leverages a patient’s own diagnostic information to map prostate cancer including extent of disease, and produces a cancer probability map with optimal treatment margins. This AI-enabled mapping platform then enables a doctor to treat locally in a doctor’s office under local anesthesia, thereby preserving quality of life.
Avenda Health is a company that creates personalized prostate cancer treatment. Its cloud-based platform, iQuest, leverages a patient’s own diagnostic information to map prostate cancer including extent of disease, and produces a cancer probability map with optimal treatment margins. This AI-enabled mapping platform then enables a doctor to treat locally in a doctor’s office under local anesthesia, thereby preserving quality of life.
Now, the company says it will be able to create even more precise treatments as it has received 510(k) clearance by the U.S. Food and Drug Administration (FDA), it was announced on Wednesday.
To use the Avenda platform, physicians upload or sync a patient’s diagnostic imaging and pathology to iQuest, which then produces a map and plan for a patient’s unique cancer. At this point, the physician can use this information to help counsel their patient and decide together on the best course of action.
For those patients who are to receive focal therapy, they then generate a treatment plan which is synced with its ablation system, called FocalPoint, prior to the therapy. During treatment, the physician guides the ablation needle under ultrasound and image fusion to the planned locations. Finally, a sensor needle is inserted into the prostate and monitors treatment feedback in real-time.
At the 2022 American Urological Association annual meeting, a retrospective study of 50 patients showed iQuest improved tumor encapsulation over conventional treatment planning from 56 percent to 80 percent.
This news comes after Avenda raised a $10 million round of funding led by VCapital, with participation from Plug & Play Ventures and Wealthing VC Club, bringing its total funding to $16 million.
“We are excited about the potential to unlock precision care in prostate cancer with iQuest, as it is a key enabling technology for focal therapy to be a reality for urologists and patients,” Dr. Shyam Natarajan, co-founder and CEO of Avenda Health, said in a statement.
“In order for a doctor to treat focally, they need to know where cancer is and the healthy tissue to avoid. This is vital information that iQuest now provides. This is a huge step forward in transforming the standard of care in prostate cancer and brings us that much closer to offering effective therapy that preserves quality of life to providers and patients across the U.S.”
(Image source: avendahealth.com)