Eko Health raises $41M to build out its AI-based smart stethoscopes
The company is looking to expand globally into the EU, South Korea, Japan, and Singapore
 Cardiovascular and pulmonary diseases are among the leading causes of death worldwide, with millions of patients unaware of their risk factors, often due to limited access to effective detection tools.
Cardiovascular and pulmonary diseases are among the leading causes of death worldwide, with millions of patients unaware of their risk factors, often due to limited access to effective detection tools.
Eko is a smart stethoscope company that uses AI to help providers identify early signs of cardiovascular disease and this week it announced a $41 million Series D round of financing.
The investment came from Double Point Ventures in the U.S., Singapore-based global investor EDBI (the corporate investment arm of the Singapore Economic Development Board), and LG Technology Ventures, backed by the LG Group of South Korea. ARTIS Ventures, Highland Capital Partners, NTTVC, and Questa Capital also participated. This round, which is its first funding since raising a $30 million Series C in 2022, brings its total funding to $165 million.
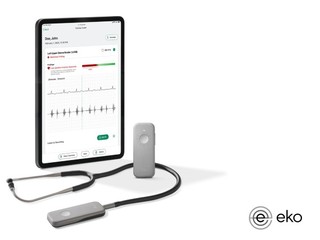
Founded in 2013, Eko has developed devices that can be used to better hear heart and lung sounds, after which its machine learning algorithms interpret that data and provide better insights. One of those algorightms includes a Low EF detection AI, which can use to detect low EF in 15 seconds during a routine physical examination.
The company has three stethoscopes it sells to individual clinicians, hospitals, departments and health systems. Its first device is the CORE stethoscope, which amplifies sound up to 40X, and can also capture, save and share sounds and can be connected to a smart device via Bluetooth. The second is the DUO stethoscope, which can be used in the clinic or by patients at home; it's an ECG and digital stethoscope combined, and made to be handheld, easy to use, and very non-challenging so patients can use it.
It's third device is called the 3M Littmann CORE Digital Stethoscope, which combines its digital and software capabilities with the acoustic stethoscope from 3M.
All of Eko's stethoscopes pair with a mobile application, which will alert physicians, flag the heart sound with a low ejection fraction detection alert, at which point the next step in care would be to refer the patient for a diagnostic echocardiogram.
The funding builds on the company recently receiving FDA clearances for its structural heart murmur and low ejection fraction (Low EF) detection algorithms.
"Since our last round in 2022, Eko has more than doubled the community of healthcare professionals using our products," Jason Bellet, co-founder and Chief Strategy Officer at Eko, told VatorNews.
"We’ve launched the FDA-cleared CORE500 Digital Stethoscope and the SENSORA early detection platform, which received FDA clearances for structural heart murmur and low ejection fraction detection. We also have more than 500,000 devices deployed with physicians and providers across the globe."
The news funding round will be used to expand globally, specifically with a focus on the EU, South Korea, Japan, and Singapore, while also deepening its presence in the U.S.
"We will invest to continue making AI guided heart and lung disease detection the standard of care. We will partner with health systems to deploy SENSORA in primary care, expand our product offerings for individual providers, and help identify patients with structural heart disease and heart failure earlier," said Bellet.
"We envision AI-assisted early detection for heart disease in every stethoscope exam globally, ensuring early detection, timely treatment, and longer lives. And we will continue to develop new detection algorithms, supported by our large dataset, for rapid deployment through our connected devices worldwide."
Related News


Eko Health Chief Medical Officer Adam Saltman on VatorNews podcast

Smart stethoscope platform Eko Health launches in the UK


