With the goal of expanding access to clinical trials, Curebase raises $40M
The company added Gilead as a strategic investor
 While all the ways that COVID affected healthcare remain to be seen, the system has already begun to respond to some of the inequities and inefficiencies that were exposed during the pandemic. That includes clinical trials, which long been inefficient, expensive, and limited in the number of people who could participate.
While all the ways that COVID affected healthcare remain to be seen, the system has already begun to respond to some of the inequities and inefficiencies that were exposed during the pandemic. That includes clinical trials, which long been inefficient, expensive, and limited in the number of people who could participate.
Recently, a number of companies have emerged, including Castor, Antidote, HumanFirst, Hawthorne Effect, Medable, ObvioHealth, and Florence Healthcare, all of which are looking to decentralize clinical trials in order to make them more efficient, and to open them up to a broader segment of the population.
That also includes Curebase, an end-to-end clinical trial platform that provides virtual and hybrid site capabilities, along with a software platform geared toward both patients and sponsors.
"Not everybody lives near a major research institute, which can lead to poor patient diversity. That has a huge impact: drugs get to market slower, it decreases patient diversity, and it makes it harder for patients of different socioeconomic backgrounds, as well as races and genders, to be in a clinical trial," Tom Lemberg, the company's founder and CEO, explained.
"We really do the opposite: we take the clinical trial and bring it to the patient, wherever they live. That means enabling patients to participate at home with their own doctors and with community health care providers."
On Thursday, the company announced that it raised $40 million in Series B funding led by Industry Ventures, along with new investors Acrew Capital, World Innovation Lab and Positive Sum, and existing investors GGV Capital, Bold Capital and Xfund.
In addition, the round also included a strategic investment by global biopharmaceutical company Gilead Sciences.
A patient-centric approach
Part of what separates Curebase's solution from others in the space, according to Lemberg, is that it has created an application that is easy for patients to use.
"We want it to be consumer grade and we really make clinical trials as easy as any other consumer experience, like ordering food or booking travel. We want being in a clinical trial to be incredibly simple, so we do that with great design, a really easy user experience for patients that lets them do everything they need to do in a clinical trial in a modern way," he said.
With Curebase, patients can look up a clinical trial, learn about the risks, the benefits, what the treatment is, and what it will mean to participate. They can call one of the company's virtual coordinators who can give them more information. If they decide they want to be in the trial, patoents can answer eligibility screeners, connect medical records, schedule a telemedicine call a coordinators to go through some questionnaires together, and provide consent.
All appointments, both in person with a local healthcare provider, or home appointments, in which the company dispatches someone to their home to do a blood draw, can be booked directly through Curebase. If the appointment is in person, the patient would go to a local health care provider, such as a local primary care or urgent care, or one of Curebase's local partners, rather than having to drive a long distance to go to a traditional site.
"We're not just doing home care, we are actually engaging community healthcare providers. A lot of clinical trials will require that you go in and get blood drawn or have an exam or have a drug administered, but we're working with local primary care doctors, local urgent cares, local specialists like GI physicians, and we're actually giving them our site operating system, which allows them to be in clinical trials, often for the very first time," explained Lemberg.
The company will also engage with patients for the rest of the clinical trial, with periodic reminders, as well as to pay them for their participation.
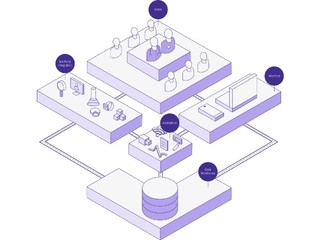
On the other side, Curebase will help the sponsors of the clinical trials, which can be a pharma company, a biotech company, or a medtech company, with finalizing the design of their protocol to make it more patient-centric. That includes configure the software platform for them, as well as recommending community sites they can use.
"We have a pretty broad range of clinical services, so we can take clinical trials from start to finish, and that makes us pretty unique in the market. Where some other companies are just providing a piece of software, we're actually providing an end-to-end experience with our clinical services team," said Lemberg.
Curebase currently has over 45 customers and it has operated approximately 60 clinical trials, mostly working in diagnostics and digital therapeutics; that includes over 10 digital therapeutics studies and over 15 diagnostics trials, in areas ranging from cancer diagnostics, women's health, and pregnancy screening.
The customers that use Curebase have been able to expand diversity in their clinical trials by roughly 32%, and more than 50% in some therapeutic areas. The company is also able to recruit over 200 patients every month to participate in these trials, while many sites are only able produce two patients total.
That recruitment speed leads to lowered costs for Curebase's customers, as does moving trials out of the traditional hospital setting, thus allowing sponsors to put that money back onto doing more research, said Lemberg.
"If each trial costs a little less, and they're spending less on administration, and monitoring and travel, then they're able to actually do more research and put more products through their pipeline," he explained.
"It varies, of course, from trial to trial, but in some studies, it can be around 10% savings if they're leveraging more home technologies, but if there's a study where they're deciding between decentralized and not decentralized, and they're deciding between whether to use 500 sites or 10 virtual sites, that could be up to a 50% cost savings."
Partnership with Gilead
Part of this funding will go toward building out the product, specifically a native mobile application, as it only currenly only exists as a web app.
It will also be used to expand globally; currently, Curebase operates clinical trials in the United States, Canada, the United Kingdom, and France. The plans going forward is to expand further into Europe, and then go to AIPAC, specifically in Japan; while Curebase is in three languages and four countries, the plan is to be in 10 languages in 10 countries by the end of the year.
Most importantly, the money will be used to go deeper into the areas of pharma and biotech; the company already has several pharmaceutical and biotech customers and now it plans to double down on those spaces, specifically with Gildead, which acted as a strategic investor in the current funding round.
"We are operating studies with Gilead. They're incredibly excited about decentralizing hybrid trials as part of their future and the investment is there to help us do some really important work together, scaling up how we operate more hybrid and decentralized trials together," explained Lemberg.
The company plans to build out features that are very unique and important to pharma and biotech customers. That includes scaling out its clinical services operation, while also providing enterprise grade data management and enterprise grade monitoring site management, and its electronic data capture systems.
This all ties into Curebase's long term vision, which is the belief that every patient, no matter where they are, should be able to be in a clinical trial at home, with their own doctor.
"People sometimes want to put a band-aid on the clinical trial problem. They say, ‘Oh, if we can just tell more patients or recruit more patients for clinical trials, if we could just let them know about the clinical trials, they'll be able to participate.’ And we've never thought that's true," said Lemberg.
"What it takes to dramatically expand access is to say, ‘you can do it with your own doctor, you can do it where you live, you can do it in a way that's convenient for you that can be just part of your normal treatment.’ That's what we need to accomplish. That's always been our mission."
(Image source: curebase.com)
Related News

Clinical trial automation platform Castor raises $12M