Digital clinical trial platform Medable raises another $78M
The company, which raised two rounds of funding in 2020, saw revenue climb by 400%
 As hard as COVID has been on the entire world over the past year, it has also been a boon for companies in the virtual care space, as the pandemic accelerated trends that were already coming, but were still years off from truly being a reality. What we now think of as "healthcare" is radically different from what it was in March of 2020.
As hard as COVID has been on the entire world over the past year, it has also been a boon for companies in the virtual care space, as the pandemic accelerated trends that were already coming, but were still years off from truly being a reality. What we now think of as "healthcare" is radically different from what it was in March of 2020.
One company that especially benefitted was Medable, a startup that digitizes clinical trials so that they can be conducted anywhere. Not only did the company onboard more than 50 new clients in 2020, and grow revenues by more than 400 percent, but it also raised two big funding rounds, first a $25 million round in May followed by a $91 million round in November.
Now Medable is back with even more funding, announcing a $78 million round on Thursday led by Sapphire Ventures, along with new investor Obvious Ventures and follow-on investment from existing investors GSR Ventures, PPD, and Streamlined Ventures. This brings's the company's total capital raised to more than $217 million.
As for why the company raised another found so quickly, it "was primarily an insider round," where the company's own investors wanted to put in more money, Dr. Michelle Longmire, CEO and co-founder of Medable, told me.
We weren’t actively looking for money, but our investors saw the fast growth we were experiencing and wanted to re-invest. This puts more wind in our sails to scale up for customers and optimize at our full commercial potential," she said.
In a blog post, Tina Hoang-To, partner at Obvious Venture, wrote about why her firm decided to invest in Medable, noting that "the team and product stood out immediately."
"There is no one size fits all for trials. A cancer trial will have very different requirements from a cardiovascular trial — different recruiting, tests, processes, check-ins, hardware, etc. Pharma sponsors need a DCT solution that they can trust to provide the same standard and quality across any type of trial, which is why sponsors trust Medable," Hoang-To wrote.
"Guided by Michelle’s expertise and vision, Medable has assembled a team of experts across therapeutic areas from leading companies such as Covance, ERT, and AstraZeneca. Medable isn’t just a technology company — it’s a partner for adopting virtual or hybrid clinical trials."
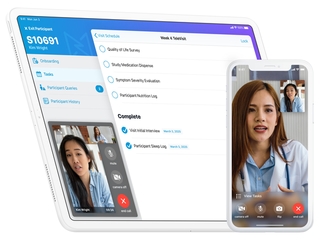
Founded in 2015, Medable's platform offers its clients, which include clinical trial sponsors, such as pharma and biotech companies, as well as clinical research organizations who manage trials for those companies, features such as patient identification and site selection through AI and machine learning; digitized patient enrollment; and the ability to do trials in any country and in any language. The company also also provides them with remote patient monitoring, and the ability to see streaming data and patient data in real-time.
Medable’s platform has been used for clinical trials in more than 60 countries, and in over 40 languages. Its customers see 3X faster enrollment and over 90 percent retention rates as a result of using the platform.
Along with its rapid growth in 2020, Medable also launched five new products last year, including a new TeleVisit mobile application last March, which was launched with its partner PPD. Other remote and mobile applications included TeleConsent, which enables fully remote informed consent and re-consent for clinical trials, and TeleCOA, which combines electronic Clinical Outcome Assessments (eCOAs) with TeleVisits to enable critical clinical trial outcomes to be captured from a patient’s home.
"The new apps enable patients to get clinical advice, capture informed consent and re-consent, and share outcome assessments from the comfort of a patient’s home, anywhere in the world," Longmire explained.
Medable says it will use the funding to continue adoption of digital trials and patient-centric strategies at global scale. Some of the key focus areas for the company going forward include internal initiatives related to ubiquitous research access, patient data fluidity, and intelligent monitoring of patients and clinical trial data.
"We’re continuously adding new features and new capabilities to our platform. We’ll use the funding to invest in both software and services that make clinical trials more accessible for patients globally, regardless of geography, income, gender and race," said Longmire.
"We’re also investing in human-centered design to transform the research experience for both patients and clinicians. Ultimately, we’re trying to bring research to patients wherever they are, giving them a consumer experience that’s as easy as on-demand food delivery."
While noting that COVID-19 pushed up the timeline for digital and decentralized trial adoption by as much as five years, Longmire also admits "that there’s a lot more work to do across the industry.
"2021 will be marked by the democratization of access to research. Biopharma sponsors can now provide remote access to new investigational medicines to patients worldwide," she said.
"Looking ahead, Medable will continue to drive radical transformation through patient-centered technologies that remove traditional bottlenecks and transform key processes. We will also build out a global ecosystem that enables research anytime, anywhere and for every person. Our team is passionate about building a world with ubiquitous research access and radically accelerated drug development timelines."
(Image source: medable.com)
Related News

Digital clinical trial company Medable raises a $91M Series C